Biological Atlas of Severe Obesity
Prevalence of obesity and associated diseases, including type 2 diabetes mellitus (T2DM) and non‐alcoholic fatty liver disease (NAFLD), are increasing. Underlying mechanisms, especially in humans, are unclear. Bariatric surgery provides an efficient treatment for obesity and type 2 diabetes and a unique research platform for the study of these diseases in humans.
ABOS cohort (Biological Atlas of Severe Obesity) is a collection of metabolic tissues (liver, muscle, visceral and subcutaneous adipose tissue, intestine) and blood samples (plasma, serum, DNA) collected as part of an ongoing clinical trial on patients with Body Mass Index (BMI) ≥ 35 kg / m2 who had undergone bariatric surgery. Blood samples and associated data are collected at 3, 12, 24 and 60 months after bariatric surgery.
The objective of ABOS cohort is to allow the identification and validation of new molecular biomarkers associated with obesity and its comorbidities, essentially Type 2 Diabetes Mellitus and Nonalcoholic Steatohepatitis (NASH).
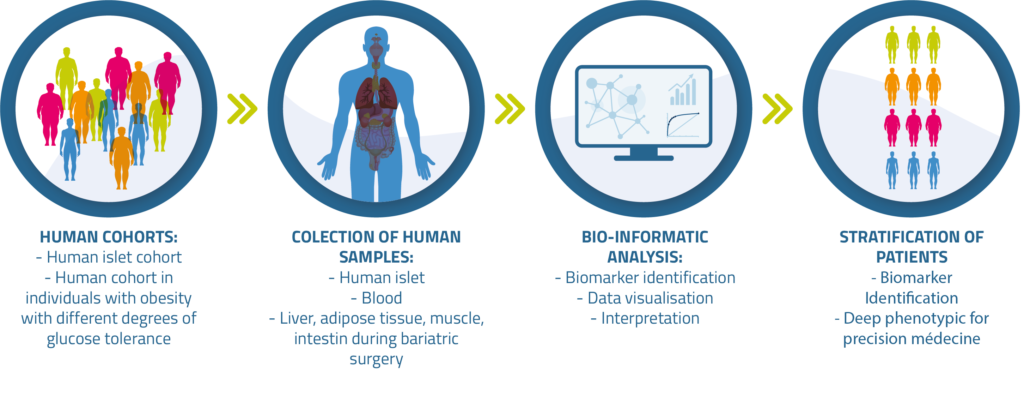
ABOS cohort - A unique collection of annotated biological samples for translational research:
To foster translational science, a prospective cohort study with annotated biological samples (ABOS) conducted by Professor François Pattou was initiated in 2006 at Lille University Hospital.
The study design includes a comprehensive clinical and biological phenotype and serum/plasma samples collected at baseline, and at 3, 12, 24 and 60 months after surgery.
Multiple tissue samples, including liver, liver, muscle, visceral and soucutaneous adipose tissue, intestin and DNA have been also collected from each participant at the time of surgery.
One of the assets of the ABOS collection is the quality and homogeneity of samples and data available, resulting from the rigorous and homogenous standard operative procedure (SOP) that were enforced since the inception of the study, during data and sample collection.
DNA, serum and tissue samples from ABOS cohort have been already utilized in several international consortia (MAGIC, IMI DIRECT, IMI RHAPSODY, IMI SOPHIA), resulting in > 80 publications in high impact journals (Nature, Nature Genetics, Nature Communications , Journal of Clinical Investigation, Gastroenterology, Hepatology, Circulation…)












